Thanks to our friends at Mad Science, here are some at-home experiments that your family can do with ingredients that you most likely already have in your house! Check out these below and find even more by visiting the Mad Science website with step-by-step instructions for each.
Thanks to our friends at Mad Science, here are some at-home experiments that your family can do with ingredients that you most likely already have in your house! Check out these below and find even more by visiting the Mad Science website with step-by-step instructions for each.
Balloon Bond
What You Need:
- 2 Small flexible plastic cups
- Balloon
What You Do:
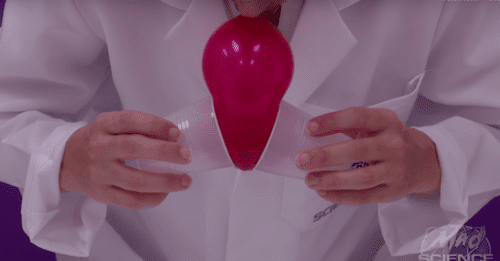
- Step 1: Hold the neck of the balloon to your mouth with one hand. Place the round end of the balloon into a small plastic cup.
- Step 2: Inflate the balloon while gently applying pressure by squeezing the sides of the cup. Pinch the neck closed when you are done.Step 3: Attempt to separate the cup and the balloon. What happens?
- Step 4: Deflate the balloon. Now hold the neck of the deflated balloon in your mouth, pick up a cup with each hand and press the opening of each one against the balloon. Try inflating the balloon between the two cups while gently squeezing their sides. Pinch the neck closed when you are done.
- Step 5: Try to pull the two cups off the balloon. What happens?
What’s Going On:
There is air all around us. It is the Earth’s atmosphere. This air pushes on us, and we call this push “air pressure”. When your balloon is small, it traps a pocket of air inside the cup. As your balloon gets bigger, the air pocket inside the cup gets bigger too, but no more air molecules can get in. This means that the air pressure inside the cup becomes lower than the air pressure outside the cup. Air always flows from an area of higher pressure to an area of lower pressure. We think of this as “suction”, but really, it is the air pressure pushing the cup against the balloon. That is why it is hard to pull them apart. No air molecules can get in as long as there is a seal between the balloon and cup. If you twist the balloon, you can break this seal, and then you can easily pull them apart!
Now Try This:
Try adding more cups as you inflate the balloon! You can do this by gently squeezing the sides of the cups, pressing the cup openings onto the balloon, and then slowly releasing the sides. See how many cups you can attach to the balloon!
Video: Click here to watch a video of this experiment
Copycat Solution
What You Need:
- Vanilla extract
- Paintbrush
- Liquid dish detergent
- Tablespoon
- 2 sheets of white paper
- Small cup
- Black ballpoint pen
What You Do:
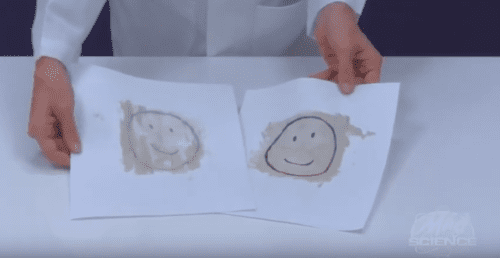
- Step 1: Use the pen to draw a picture on a sheet of paper. Make the lines of the drawing as thick as possible.
- Step 2: In the cup, mix one tablespoon of vanilla extract with one tablespoon of liquid dish detergent.
- Step 3: Use the paintbrush to paint a thin layer of the vanilla extract and detergent mixture over your drawing.
- Step 4: Cover your drawing with the second sheet of white paper.
- Step 5: Use the back of the spoon to press down on the top sheet. Gently rub your drawing by moving the spoon in small circles.
- Step 6: When you can see your drawing through the paper, peel off the top sheet.What do you see?
What’s Going On:
You copied your drawing! The detergent in your mixture binds to the ink in your picture. The ink mixture transfers to the second page when you press the sheets together. If you draw thick lines and use lots of ink, you can make more than one copy.
Now Try This: Try drawing with different kinds of markers or different colored pens. Does the copycat solution work for these inks?
Video: Click here to watch a video of this experiment
Ghost Bubbles

What You Need:
- Corn syrup
- Dish soap
- Jar
- Marker
- Chenille stem (pipe cleaner)
- Spoon for mixing
- Water
- Measuring Cup
- Safety Note: Do not drink the mixture.
What You Do:
- Step 1: Pour 250mL (1 cup) of dishwashing soap, 125mL (½ cup) of corn syrup, and 62.5mL (¼ cup) of water into the jar.
- Step 2: Slowly mix the three liquids together with the spoon. Do not mix quickly, otherwise bubbles will form.
- Step 3: Wrap a chenille stem end around a marker to make a bubble wand. Slide the chenille stem off the marker, and twist the stem on itself.
- Step 4: Dip the wand loop into the solution. Wait for five seconds the first time you do this to let the bristles soak up the solution.
- Step 5: Remove the wand from the solution and blow through the loop. What do you see? What happens when the bubbles pop?
What’s Going On:
Water molecules are attracted to each other. They pull on each other. When water meets air, the water molecules stick together in a layer at the surface. This is because they are more attracted to each other than to the air molecules. We call this surface tension. Normal water has too much surface tension to make bubbles. Adding a detergent like dish soap weakens the surface tension so bubbles can form. When the water in a bubble dries up, or evaporates, the bubble bursts. Corn syrup slows down this process, so the bubbles are stronger and last longer. When the water finally does dry up, the bubble pops, leaving a ghostly film of corn syrup and soap.
Now Try This:
Try making bubble solutions with different ratios of corn syrup, dishwashing soap, and water. Do the bubbles last longer? Are the bubbles as strong? Does the bubble solution become stronger or weaker over time?
Video: Click here to watch a video of this experiment
For many more fun experiments and science resources, please visit the Mad Science Website and Facebook page.